The molecular structure and absorption spectrum of hydroxy substituted dibenzoylmethanatoboron difluoride in solution: A theoretical and experimental study
By Natalia Gelfand, Alexandra Freidzon, Elena Fedorenko
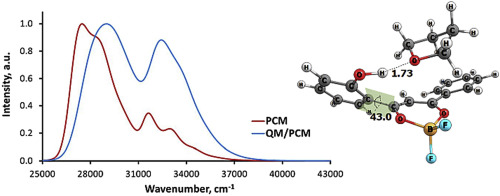 Electronic spectroscopy and quantum chemistry are used to study the structure and absorption spectra of the hydroxy substituted dibenzoylmethanatoboron difluoride (OHDBMBF2) in solutions. Introducing a hydroxy group in the diketonate moiety allows the dye to form intermolecular complexes with proton acceptors, such as solvents or analytes, thus making it a promising chemical sensor. Our calculations show that donor oxygen-containing solvents break the intramolecular hydrogen bond OH···Odik and form an intermolecular OH···Osolv bond thus disrupting the coplanarity of the dye and affecting the position and shape of its absorption bands. The spectra calculated with explicit solvent combined with polarizable continuum model (PCM) better agree with the experiment than those calculated only within PCM.
Electronic spectroscopy and quantum chemistry are used to study the structure and absorption spectra of the hydroxy substituted dibenzoylmethanatoboron difluoride (OHDBMBF2) in solutions. Introducing a hydroxy group in the diketonate moiety allows the dye to form intermolecular complexes with proton acceptors, such as solvents or analytes, thus making it a promising chemical sensor. Our calculations show that donor oxygen-containing solvents break the intramolecular hydrogen bond OH···Odik and form an intermolecular OH···Osolv bond thus disrupting the coplanarity of the dye and affecting the position and shape of its absorption bands. The spectra calculated with explicit solvent combined with polarizable continuum model (PCM) better agree with the experiment than those calculated only within PCM.