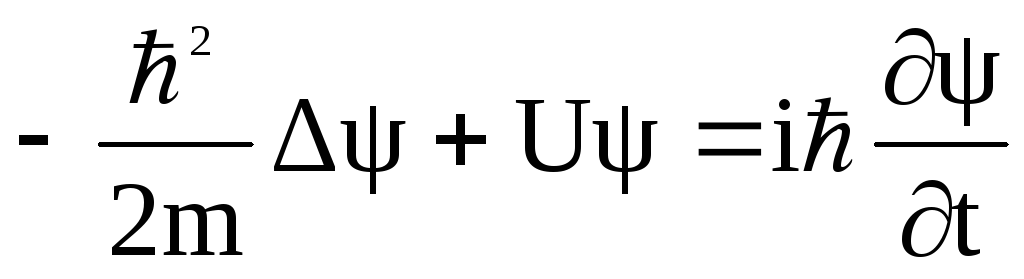Specific Features in the Triplet–Triplet Absorption Spectra of Multi-Chromophoric Molecules: a Computational Study
By M. V. Alfimov, I. A. Anger, N. O. Dubinets, and A. A. Bagaturyants
Singlet–singlet (S–S) and triplet–triplet (T–T) absorption spectra of multi-chromophoric molecules containing polyphenyl fragments are studied computationally by quantum chemical DFT/TDDFT methods. Patterns observed previously in experimental absorption spectra of the parent chromophore and its composite analogs are interpreted based on the localization of molecular orbitals (MO) involved in the optically active transitions. In the singlet ground state, the tilt between the phenyl fragments in the parent molecule and in the respective fragments of its composite analog is similar. The composite molecules exhibit symmetric geometry; therefore, MO localized on different ‘parent’ moieties are quasi-degenerate. Optically active transitions are delocalized over these moieties, and the position of the intense S–S absorption band is not altered. By contrast, the equilibrium geometry of the triplet ground state of the parent molecule is planar. In the multi-chromophoric analog, only one of the ‘parent’ polyphenyl moieties becomes planar. This property results in two specific features: (i) the character of the lowest triplet state is similar in the parent chromophore and its composite; therefore, no shift in their phosphorescence spectrum is observed; (ii) a change in the symmetry of the composite analog gives rise to intramolecular charge-transfer (CT) bands in its T–T absorption spectrum, which is not observed in the spectrum of the parent chromophore. An increase in the conjugation length of the parent chromophore leads to a decrease in the energy difference between the local and intramolecular CT bands in the spectrum of the composite species. These features are reproduced at the DFT/TDDFT level of theory with a good agreement with experimental data.