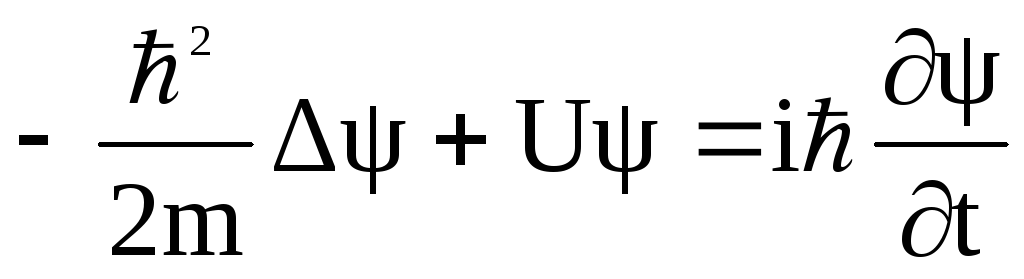Study of the behavior of the 4-DASPI dye in samples of silicate hydrogel by fluorescence spectroscopy and quantum chemistry
By Anna Medvedeva, Nikita Dubinets, Alexander Koshkin, Elena Rykova
The behavior of the 4-[4-(dimethylamino)styryl]-1-methylpyridinium iodide (4-DASPI) dye in samples of silicate hydrogel obtained from tetrakis(2-hydroxyethyl) orthosilicate with the dye added at the synthesis stage or adsorbed on a previously synthesized hydrogel substrate is studied by luminescence spectroscopy and molecular dynamics and quantum chemistry calculations. Small hypsochromic shifts of the fluorescence spectra of the dye upon its interaction with silica are observed. Energies of adsorption of 4-DASPI in the ground and excited state on two types of amorphous silica are calculated and contributions of Coulomb and dispersion interactions are estimated. A transition of the 4-DASP+ cation to the TICT state upon its excitation in water and methanol solutions is proved by TD DFT calculations. Partial twisting of the 4-DASP+ cation on silica, both in the pore and on the surface, is proposed based on the results of calculations and experimental measurements. The main difference between the samples synthesized with the introduction of 4-DASPI into the reaction mixture and by the gel synthesis followed by the adsorption of 4-DASPI seems to be capturing of the dye molecule in a pore in hydrogel with freezing molecular vibrations and rotations and, consequently, the growth of fluorescence intensity in the first case and the adsorption of the dye on the silica surface with relatively easy molecular vibrations and hindered rotations in the second case. This explains a strong difference in the intensities of fluorescence bands in the spectra of the studied samples.