Conformations and Optical Transitions of the BODIPY Dye Dimer with a Siloxane Spacer
By N. O. Dubinets, D. S. Ionov, Yu. N. Kononevich and A. A. Pakhomov
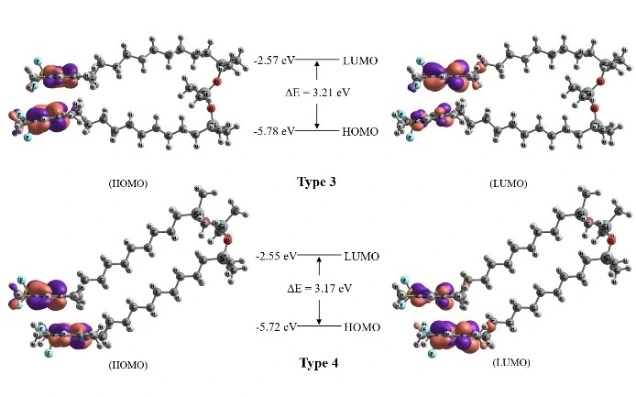
In this communication, the results of theoretical investigations of a 1,3,5,7-tetramethyl-BODIPY derivative (TMB) and a dimeric molecule composed of two TMB fluorophores linked by a flexible siloxane spacer (di-TMB) in various solvents are presented. The existence of two energetically close configurations for the di-TMB molecule is revealed. The analysis of the patterns of frontier molecular orbitals indicates that the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are predominantly localized on the chromophores. Furthermore, in the di-TMB molecule, a nearly complete transfer of electron density occurs between the chromophores.